Separation Techniques
-magnetic attraction
-filtration
-evaporation to dryness
-crystillation
-distillation
-chromatography
-sublimation
The physical methods used will actually depend on the nature of the constituents.
Separating Funnel
-for 2 immiscible liquids
-ex:oil and water
Filtration
-for insoluable solids with liquids( suspension )
-the insoluable solids that remain on the filter paper are called residue,whereas the liquids that pass through the filter paper are called filtrate
-Ex: air filters in air conditioners to remove solid impurities from the air
hair in our nostrils to trap dust in the air
Evaporation to dryness
-Ex: obtaining salt from seawater
Distillation
-to separate a solvent from a solid-liquid solution or liquid-liquid solution.
-pure water can be distilled from soft drinks
Fractional distillation
-used to separate miscible liquids with different boiling points.
-the liquid with lower boiling points will vapourise first
Ex: alcohol and water
Chromatography
-to separate the different components in a liquid orgaseous mixture
-Ex:used to separate the different coloured components that make up black ink
-happens because some components of the liquid mixture travels at a faster pace than other components on the paper or any absorbent material
-the mixture is separated as the solvent travels up the paper
-a chromatogram of the separated components is obtained
-advantage: able to obtain the results quickly
only a small concentrated amount of sample is required for chromatography
Reflections
After learning all the separating techniques and gone through all the experiments, I had discovered more separating techniques as compared to primary school. I had also knew the steps to carry out the methods.
These separating techniques is very useful , especially in surviving skills. For example, we can obtained pure water to drink from muddy water through filtration.
Tuesday, August 31, 2010
Lesson learnt on solute,solvent,suspension and solution
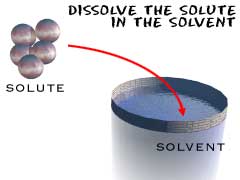
Solute is a substance which dissolves in a liquid.
Solvent is a liquid which dissolves other substances.
Solution is when a solute dissolves in a solvent.It is a homogeneous mixture in which one or more substances are dissolved in another substance.
Remember, solute + solvent = solution!!
Question : 30 cm ( cube ) + 50 cm (cube ) equals to 80 cm ( cube )?
False!!!!!!!
That is not true as the solute particles are smaller then the solvent particles, therefore the solute particles will occupy the space between the solvent.
When a solute dissolves in a solvent, the solute particles surround each solvent particles. The particles then mix uniformly to form the solution. They do not combine chemically.
Types of solution
solid-liquid: sugar solution, salt solution
gas-liquid:hydrochloric acid, soft drinks
solid-solid:brass, bronze
liquid-liquid:vinegar, beer, wine
gas-gas:air,natural gas
Suspension
-suspension is a mixture of small insoluable particles in a liquid gas.
-when a solid does not dissolve in a liquid, a suspension is formed.
-the solid is said to be insoluable
Ex of suspension: calamine lotion, medicine, sea water
Question: Why does suspension formed?
Ans: In a suspension, the solvent particles are smaller, whereas the solute particles are bigger, therefore they don't formed a solution.
Question:Why does suspension appears cloudy or chalky?
Ans: It's because some particles are so light that they do not sink but remain floating.
As the solid particles are bigger than the liquid particles and the holes in the filter paper, suspension can therefore be separated by filtration.
A saturated solution contains the maximum amount of solute that can dissolved in a fixed amount of solvent at a particular temperature.
Solubility of solutes
-the maximum amount of solute that can dissolved in 100g of solvent at a given temperature.
-higher the solubility, the more soluble the substance.
Strength of a solution=concentration
The concentration of a solution=the amount of solute in 1000cm ( cube ) of solution.
A concentrated solution can be made more diluted by adding more solvent.
Ex: to dilute a cup of orange juice, we can add more water
Factors affecting the extent of dissolving
-Nature of a solvent
The solubility of a solute is different in different solvents.
-Nature of solute
Different solutes have different solubility in a solvent.
-Temperature
The higher the temperature of solvent, the higher the solubility of solute.
Rate of dissolving is affected by
-Temperature
-Rate of stirring
-Size of solute particles
Reflections
After learning all these, I had more understanding of liquids and solids particles and they can even help me in my daily life.
Foe example, when I'm about to be late for school, I would prepared a cup of milo with high temperature of the water and also stir as fast as possible, so that the solute particles will dissolved faster and I will not be late.
I could also used this concept when making a cup of orange juice or even Ribena. I learnt how to make the juice more diluted or concentrated according to the guest.
Monday, August 30, 2010
Lesson learnt on element,compound

Elements are substances that cannot be broken down into simpler substances by chemical methods.
The different elements in a compound always join together in a fixed proportion by mass.
However, compounds are substances that could be broken down but it can only be broken down by chemical methods.
All the elements can be found in the Periodic Table. The columns of the Periodic Table are called group whereas the rows are called period.
Name of the element Symbol of the element
Aluminium Al
Zinc Zn
Copper Cu
Magnesium Mg
Mercury Hg
Iron Fe
Carbon C
Sulfur S
Chlorine Cl
Nitrogen N
Iodine I
Hydrogen H
Oxygen O
For those that were highlighted in red, they need a " 2 " below them when the questions did not mention the word element.
Elements are broken down into metals and non-metals.
Metals:Aluminium,calcium,copper,mercury,iron,zinc
Non-metals: Oxygen, hydrogen,helium,iodine,sulfur
Differences between metallic element and non-metallic element
Metallic element Non-metallic element
High melting point Low melting point
High boiling point Low boiling point
Good heat conductor Poor heat conductor
Good electrical conductor Poor electrical conductor
Ductile,malleable Brittle
High densities Low densities
Sonorous Not sonorous
Uses of some elements
Carbon
-exists in diamond,graphite,soot,charcoal
Diamond
-hardest
-attached to drills or saws for cutting concrete and drilling into rocks
Nitrogen
-unreactive element
-used in packaging of food
-used to make fertilisers
Sulfur
-non-metallic, yellow
-used to harden rubber used for making tyres
-used in pesticides and drugs
Iodine
-non-metallic
-sublimes when heated to form a violet vapour
-forms tincture of iodine as an antiseptic when dissolved in ethanol
Zinc
-coat iron sheet to make galvanised iron
-mixed with copper to form alloy, brass
Mercury
-the only metallic element that is a liquid at room temperature
-used in thermometers, fluorescent lamps and some dental fillings
Magnesium
-used to make fireworks
Reflections
Because of all these lessons I learnt, I could then be more aware of the elements around me and their uses. For example, if I ever had the chance to see magnesium, I would be more cautious and it was used to make fireworks and thus flammable.
I could also applied this to my daily life and after learning these concept, I could be more aware of the surroundings and the different types of elements.
Friday, August 27, 2010
Self reflection
I think back about my lessons on Science and thought to myself: Have I really learnt and gained some knowledge?Can I list out knowledge that I learnt,without looking at my notes?
The answer?Some yes and some no.
I reflect deeply,and realise that I had indeed learnt some very useful knowledge, such as knowing more about my body tissues, and also the fact that we should not put plants in our bedroom at night.This is because they will compete with us for oxygen at night when the plants are not making food.
However,I think that instead of copying notes and learning the notes or by blogging, I should also take the initiative to read through the notes clearly and ask myself questions,such as why would this happen?
I could then asked teacher about my questions and maybe shared with everyone.This way, I would not only learnt blindly, but learnt with understanding.
The answer?Some yes and some no.
I reflect deeply,and realise that I had indeed learnt some very useful knowledge, such as knowing more about my body tissues, and also the fact that we should not put plants in our bedroom at night.This is because they will compete with us for oxygen at night when the plants are not making food.
However,I think that instead of copying notes and learning the notes or by blogging, I should also take the initiative to read through the notes clearly and ask myself questions,such as why would this happen?
I could then asked teacher about my questions and maybe shared with everyone.This way, I would not only learnt blindly, but learnt with understanding.
Thursday, August 26, 2010
Readings
A ruler's reading is to 0.1 cm
A vernier calipers's reading is up to 0.01cm
A micrometer's reading is also up to 0.01cm
On the vernier calipers, the reading on the top will be called the main scale, while the one at the bottom will be called vernier scale.
When we count the reading on the main scale,we will round it off to 1 decimal point.When we count the reading on the vernier scale,we round it off to 2 decimal points.
On the micrometer,the left is called sleeve while the right is called thimble.
When there's an X positive zero error, we minus X from the observed reading and vice versa.
Measurements
micro = 1 millionth
milli = 1 thousandth
centi = 1 hundredth
deci = 1 tenth
kilo = 1 thousand
mega = 1 million
1cm = 10 mm
1m = 1oocm
1 km to the power of 3 = 1000 000 000 m to the power of three
1 cm to the power of 3 = o.ooooo1m to the power of 3
1mm to the power of 3 = 0.000000001 m to the power of 3
Volume of a cube = L to the power of 3
Volume of a rectangular block = L x B x H
Volume of a sphere = 4
---- pie x radius x radius
Volume of a cylinder = pie x radius x radius x height
A vernier calipers's reading is up to 0.01cm
A micrometer's reading is also up to 0.01cm
On the vernier calipers, the reading on the top will be called the main scale, while the one at the bottom will be called vernier scale.
When we count the reading on the main scale,we will round it off to 1 decimal point.When we count the reading on the vernier scale,we round it off to 2 decimal points.
On the micrometer,the left is called sleeve while the right is called thimble.
When there's an X positive zero error, we minus X from the observed reading and vice versa.
Measurements
micro = 1 millionth
milli = 1 thousandth
centi = 1 hundredth
deci = 1 tenth
kilo = 1 thousand
mega = 1 million
1cm = 10 mm
1m = 1oocm
1 km to the power of 3 = 1000 000 000 m to the power of three
1 cm to the power of 3 = o.ooooo1m to the power of 3
1mm to the power of 3 = 0.000000001 m to the power of 3
Volume of a cube = L to the power of 3
Volume of a rectangular block = L x B x H
Volume of a sphere = 4
---- pie x radius x radius
Volume of a cylinder = pie x radius x radius x height
SI
SI stands for System International of Units
Acceleration: change of speed
----------------
time
Units of acceleration: m/s
----
s
= m
-- divide by s
s
= m 1
-- times -----
s s
=m/s to the power of 2
SI unit of density = kg
---
m to the power of 3
=kg/m to the power of 3
Reflections
After learning all the readings, I find it useful especially when I need it in the future in Maths.This is also useful if my jod were to involve calculationand measuring.Through the experiment, I had also experience and know how to use the different types of instruments.
Acceleration: change of speed
----------------
time
Units of acceleration: m/s
----
s
= m
-- divide by s
s
= m 1
-- times -----
s s
=m/s to the power of 2
SI unit of density = kg
---
m to the power of 3
=kg/m to the power of 3
Reflections
After learning all the readings, I find it useful especially when I need it in the future in Maths.This is also useful if my jod were to involve calculationand measuring.Through the experiment, I had also experience and know how to use the different types of instruments.
Graph
Graph- x axis vs y axis
Important Notes
-against = VS
-always include the units,eg: .../min , .../km
-x represent the value
-every graph must have a TITLE
-TITLE must be underlined
-must be drawn in pencil
Eg: Plot a graph of the speed of the different cars against the time taken by the cars to reach the finishing point
X axis (vertical) : speed of the different cars
Y axis ( horizontal) : time taken by the cars to reach finishing point
Important Notes
-against = VS
-always include the units,eg: .../min , .../km
-x represent the value
-every graph must have a TITLE
-TITLE must be underlined
-must be drawn in pencil
Eg: Plot a graph of the speed of the different cars against the time taken by the cars to reach the finishing point
X axis (vertical) : speed of the different cars
Y axis ( horizontal) : time taken by the cars to reach finishing point
Subscribe to:
Posts (Atom)

